Editor’s Note (11/1/23): Pig heart transplant recipient Lawrence Faucette died on October 30, according to the University of Maryland Medical Center, where Faucette received the surgery and posttransplant care. The medical team reported that Faucette showed signs of organ rejection. He had lived for nearly six weeks after receiving a genetically modified pig heart.
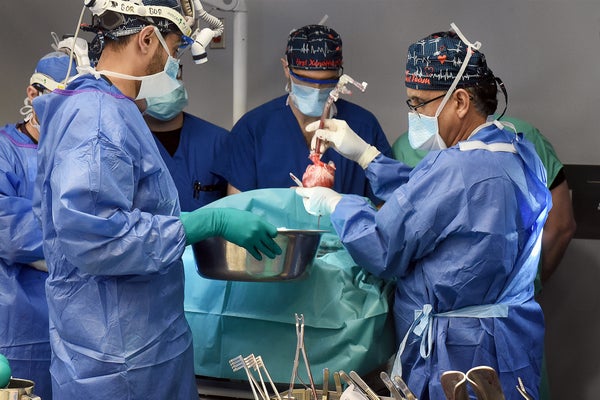
Late last month a team of researchers at the University of Maryland School of Medicine transplanted a genetically modified pig heart into a person—the second such surgery ever attempted—and it has kept him alive for the past few weeks. The patient, 58-year-old Lawrence Faucette, underwent the highly experimental procedure under a “compassionate use” pathway, in which the U.S. Food and Drug Administration permits an unapproved therapy when a person is seriously ill or dying and has no other options available. Faucette was not eligible for a conventional human heart transplant because he had peripheral vascular disease and other complications, which narrowed the outlook for success.
By mid-October, Faucette was continuing to recover and doing physical therapy. “He’s had a rough time,” however, Bartley Griffith, a surgeon at the University of Maryland, who performed Faucette’s procedure as well as the previous one, said at that time. According to Griffith, Faucette was living at home when the FDA first approved the surgery, but he was subsequently hospitalized with fluid in his lungs. Then he suffered a cardiac arrest the night before the surgery. Still, he had so far responded well to the transplant—and was sitting up in a chair two days afterward.
More than 100,000 people are waiting for an organ transplant—most of them for kidneys—so researchers have long been exploring xenotransplantation: transplanting other species’ organs into humans. To prevent the human immune system from attacking these alien organs, scientists have begun to breed genetically modified donor pigs that lack certain genes or have other genes added.
In the past couple of years, pig xenotransplants have been tested in both nonhuman primates and deceased humans—but the ultimate goal is to conduct human clinical trials on a bigger scale. The results of the recent compassionate use transplant will likely influence the FDA’s consideration of whether and when to allow such trials to take place. Many researchers hope this could happen in the next year or two.
“I would love to see heart [xenotransplantation in] a clinical trial next year and kidney [xenotransplantation trials] shortly thereafter,” says Jayme Locke, director of the division of transplantation at the University of Alabama at Birmingham, who was not involved in the latest experimental surgery. Locke and her colleagues have performed several kidney xenotransplants in humans who had suffered brain death. “The FDA holds those cards, and I think it’s going to really depend on what their risk tolerance threshold is,” she says. “But I’m hopeful. I think the FDA wants to see this happen.”
In January 2022 Griffith and his team at the University of Maryland transplanted a genetically modified pig heart from the company Revivicor into a patient, David Bennett, Sr., who lived for two months before the heart failed, and he passed away. The heart was later found to be infected with a pig virus that had escaped screening, although other factors may have also played a role in the transplant’s failure and Bennett’s death.
“We took a pretty good swing at the ball the first time, and we got very close to a prolonged success, we think,” Griffith says. There were some unforeseen circumstances in that first xenotransplant that may have affected its result, such as the pig virus that was later found in the heart, Griffith adds. Since then his team and others have developed better methods to test for these viruses.
Bennett’s family is glad he was able to take part. “He lived for two months, and we got to spend more time with him. So I was grateful for that,” says his son, David Bennett, Jr., who hopes Faucette’s surgery will be successful. “I’m thankful for every dream and hope and every person that is involved in this and the ability that it has to move forward.”
One major difference between the first and second surgeries is that although Faucette was considered terminally ill, he was much healthier than Bennett was at the time of his procedure. Unlike Bennett, Faucette had been living at home until shortly before the transplant and was much more mobile, according to Muhammad Mohiuddin, director of the Cardiac Xenotransplantation Program at the University of Maryland School of Medicine, who is managing Faucette’s anti-transplant-rejection regimen.
Other researchers agree that Faucette was a more appropriate candidate for such a novel procedure. “My overall feeling is that this patient was in much better shape than the previous patient,” says Nader Moazami, a cardiothoracic transplant surgeon at NYU Langone Health, who was not involved in Faucette’s transplant. “Part of the problem when we have a patient who is very, very sick—and you go into doing experimental xenotransplantation, where we still don’t necessarily know exactly what combination of immunosuppressive agents is good—is that those patients are very prone to developing a variety of complications.” Last year Moazami and his colleagues transplanted genetically modified pig hearts into two people who had suffered brain death, and the organs functioned well for several days.
Both Bennett's and Faucette's procedures used standard immunosuppressive drugs in addition to an experimental one. With Bennett, the team employed an experimental antibody drug called KPL-404, which blocks a receptor called CD40 that activates the host’s B cell and T cell immune responses, which can lead to the rejection of a foreign organ. With Faucette, the team used a drug called tegoprubart, which was developed by Eledon Pharmaceuticals and blocks the molecule, or ligand, which binds to CD40. Tegoprubart has been tested in phase 2 clinical trials for human kidney transplants but is not yet FDA-approved.
The team is also working with international laboratories that are using artificial intelligence to evaluate biopsies of Faucette’s heart tissue, an assessment that Griffith says could detect early signs of tissue rejection.
The FDA has been closely watching Faucette’s case, which could inform the agency’s decision to approve clinical trials of xenotransplantation. Locke thinks the first trials will likely involve hearts, not kidneys, because dialysis can keep people with kidney disease alive for several years. There is no comparable substitute for heart function. Dialysis is still an imperfect option, however, and Locke hopes kidney xenotransplants will be next. “I think there is a common misperception that dialysis is an appropriate alternative, and it is not,” Locke says. “People may live a bit longer on dialysis” than on life-extending heart therapies, she says, but dialysis can’t replace kidney function in the long term. Only transplants can do that. “We now have an alternative organ source that I genuinely believe is better than dialysis,” she says. “And it’s time to be able to test that.”