Editor’s Note (4/10/23): On Friday a federal judge in Texas ordered a hold on federal approval of the abortion drug mifepristone, which has been approved by the Food and Drug Administration for more than 20 years. The ruling is set to take effect within seven days of that order. On the same day a federal judge in Washington State ruled that no changes can be made to the drug’s availability in 17 states where abortion is legal or in Washington, D.C. This article, originally published in May 2022, describes how mifepristone works in concert with another drug, misoprostol, to end a pregnancy.
In 2016 the U.S. Food and Drug Administration approved a two-drug combination of Mifeprex (also called RU-486 or mifepristone) and Cytotec (commonly known as misoprostol) to induce abortion without surgery. In 2019 the Centers for Disease Control and Prevention reported that approximately 42 percent of all abortions in the U.S. were medication-based.
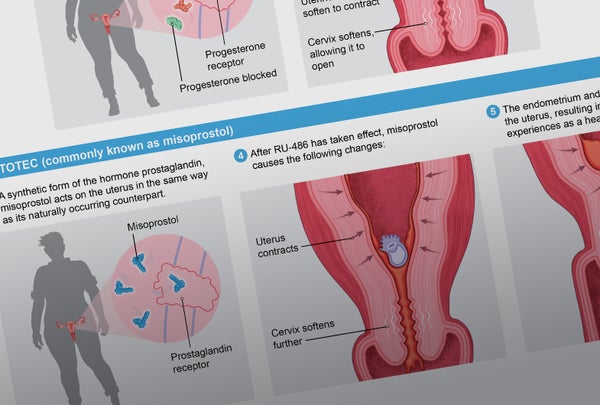
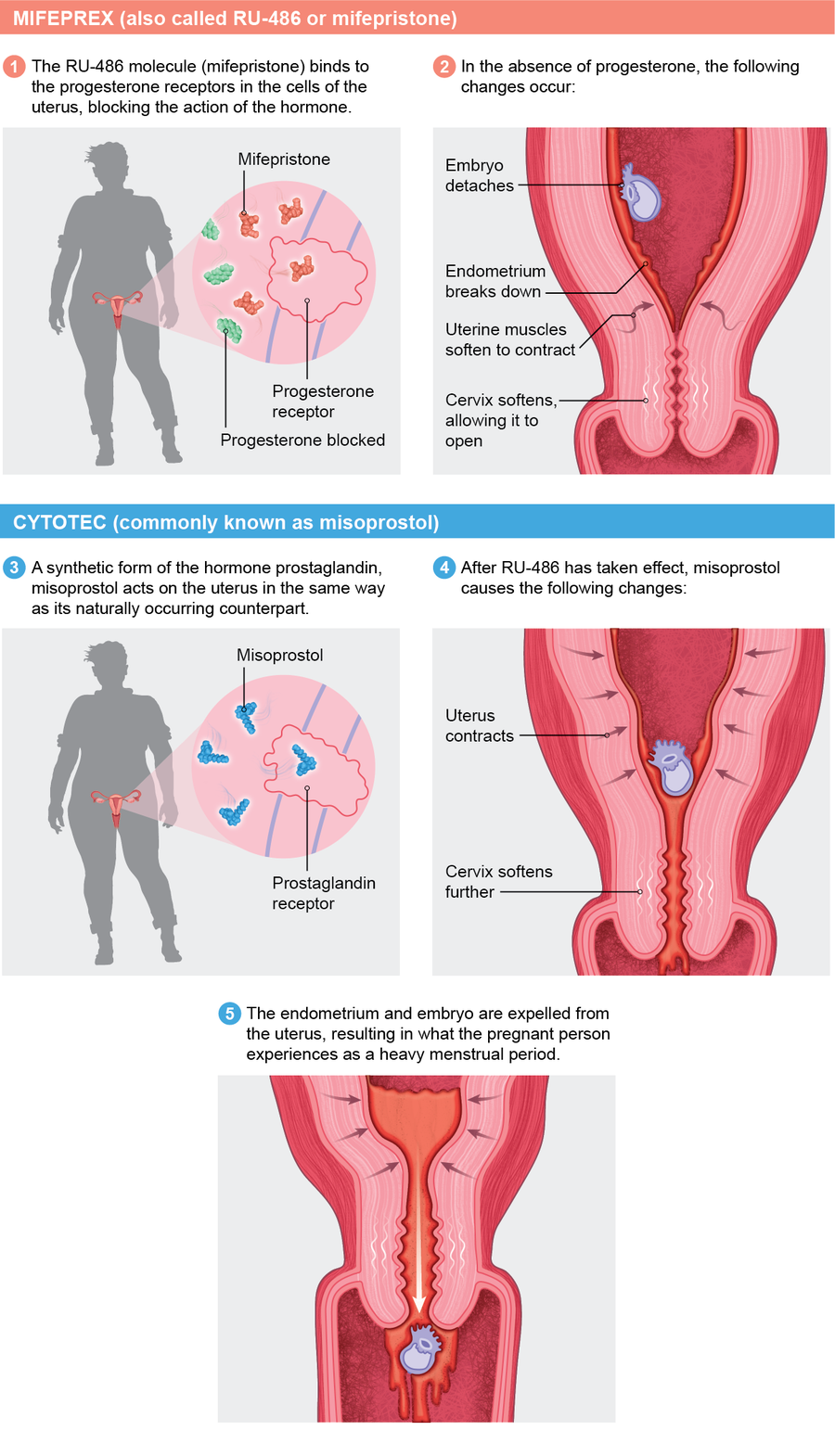
To start the process, a person takes mifepristone within 10 weeks from their last period. One or two days later they take misoprostol. Both drugs work individually, but they are more effective together. Mifepristone blocks progesterone's action on the uterus, making it incapable of supporting a pregnancy. Misoprostol, among other things, starts uterine contractions.
Research has shown medication abortion to be safe and effective. According to a 2015 study from the University of California, Los Angeles, 99.6 percent of more than 30,000 women who were seeking a medication abortion were able to terminate their pregnancies. In a review of clinical trials published in 2013, using mifepristone and misoprostol together, just 0.3 percent of the more than 45,000 women studied had complications that required hospitalization. The treatment did occasionally fail if the pregnancy was longer than eight weeks or if the instructions weren't followed. The mortality rate of the medications is less than 0.001 percent.