To melt a solid, heat it. To freeze a liquid, cool it. It's simple—except when it isn't, because quantum mechanics can flip even the intuitive logic of melting and freezing on its head.
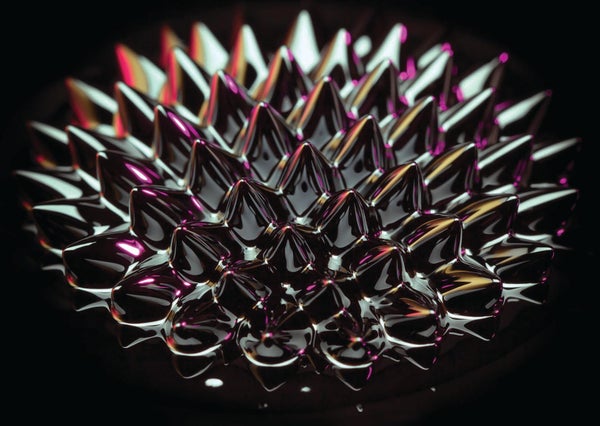
Physicists recently showed in Nature Communications how heating a quantum fluid—in this case, a very cold gas of magnetic atoms—can actually “freeze” it into an orderly state called a supersolid. This unexpected behavior was first observed in 2021, but scientists couldn't explain it until now.
“This paper manages to introduce some new theoretical description, which now successfully describes experimental observations people didn't understand before,” says physicist Tim Langen of the University of Stuttgart, who was not involved in the new study.
Quantum particles, which are both particles and waves, can be imagined as clouds of probability. The odds of finding a particle at any point in the cloud at a given moment are linked to the particle's wave behavior, described by a formula called a wave function. In a quantum fluid, particles smear together into a single entity whose collective behavior is governed by just one wave function. Typically they are also “superfluids”—they flow without friction.
Supersolids have similar properties, but they additionally have orderly, rippled structures, says study co-author Francesca Ferlaino, an experimental physicist at the University of Innsbruck and the Institute for Quantum Optics and Quantum Information, both in Austria.
In 2021 Ferlaino and her team found that warming an ultracool quantum fluid of the magnetic rare earth element dysprosium could solidify it into a supersolid's distinctive peaks. But given such an unexpected result, “we had to convince ourselves with the theory that this is actually something that makes sense,” says study co-author Thomas Pohl of Denmark's Aarhus University.
The team now shows that this counterintuitive behavior arises from a strange synergy between heat and the natural tendency of magnetic atoms to pile up.
At the atomic level, temperature is motion: it measures the energy of particles' random movements. Heating something is thus a bit like shaking it, injecting random thermal fluctuations that in this case nudge atoms out of the quantum fluid's unified, blurred-together state. Because they're so magnetic, these breakaway particles interact strongly with the quantum fluid and encourage dysprosium atoms' inherent inclination to stack. This influence changes the entire quantum fluid's wave function, pushing it into a supersolid state with regularly spaced peaks.
“How weird and counterintuitive this is—this is what I like finding as a physicist,” says study co-author Juan Sánchez-Baena of the Polytechnic University of Catalonia and Aarhus University. “If you find all the things that you are expected to find, things get boring.”