The human brain's billions of neurons represent a menagerie of cells that are among both the most highly specialized and variable ones in our bodies. Neurons convert electrical signals to chemical signals, and in humans, their lengths can be so tiny as to span just the tip of a sharpened pencil or, in some cases, even stretch the width of a doorway. Their flexible control of movement and decision-making explains why they are so key to survival in the animal kingdom.
Most animals depend on their allotment of neurons for survival. It might stand to reason, then, that the common ancestor of all of these animals also moved about the Earth millions of years ago under the guidance of electrochemical signals transmitted and received by networks of neurons. The idea that these pivotal cells evolved multiple times seems implausible because neurons are highly complex cells, and they are also quite similar among animal lineages. But a series of recent evolutionary biology studies are straining the assumption that all animal neurons have a single origin.
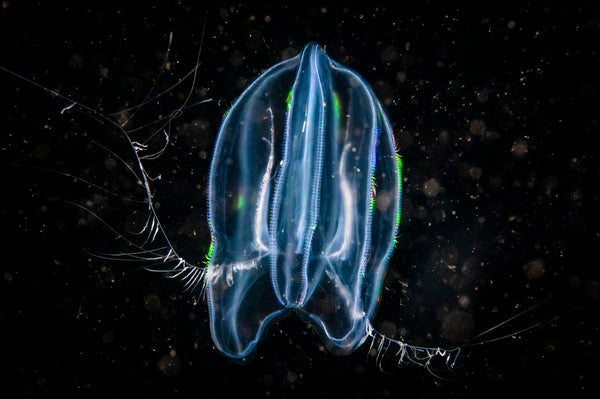
These findings are the culmination of several years’ worth of research on and debate about early evolutionary animal lineages and the cells and systems present in those species. The first such finding came from studying relationships among early animals, with a focus on two particular types of organisms: sponges (including sea sponges and freshwater varieties) and ctenophores, invertebrates often known as comb jellies, though they are unrelated to jellyfish. For roughly 15 years, evolutionary biologists have been divided over whether ctenophores or sponges were the first animals to branch from all other animals in the evolutionary tree. Hundreds of millions of years ago the common ancestor to all living animals branched into two species. On one side was the common ancestor of all groups of animals except for one. On the other side was that “one”—the “sister group” that was the first to diverge from all other animals. A persistent question has been whether the sister group was the sponges or ctenophores.
A compelling paper published last year lends strong support to the hypothesis that ctenophores are, in fact, the long-sought sister group. Ctenophores, the researchers found, branched off before sponges and are therefore the group most distantly related to all other animals. Yet despite the new evidence, what exactly happened in evolutionary history is still unsettled because of the puzzle it poses in explaining the evolution of neurons.
Neurons are absent in sponges and present in ctenophores and nearly every other animal on the planet. If ctenophores branched off before sponges in the tree of life, that suggests one of two scenarios for neuron evolution. In one scenario, the precursor to all animals, which lived nearly a billion years ago, had neurons, and every single animal species inherited them. That would mean that sponges must have lost their neurons at some point, because they no longer have the neurons that their ancestors inherited.
An alternative posits that the ancestor to all animals lacked neurons, which explains why early-diverging animals such as sponges have no neurons. Neurons in most animals, then, must have arisen later, after sponges diverged—except for neurons in ctenophores. If the common ancestor lacked neurons, and neurons in most animals arose after ctenophores and sponges had already branched off, then the neurons in ctenophores must have evolved independently. Neurons evolve twice in this scenario—once in ctenophores and then later in other animals—which calls a single origin of neurons into question.
“In the moment, I would say I’m undecided” as to which of these two scenarios is more likely, says Detlev Arendt, a professor and senior scientist at the European Molecular Biology Laboratory specializing in the evolution of neurons and nervous systems.
Max Telford, a professor of zoology at the Center for Life’s Origins and Evolution at University College London, is more supportive of the first scenario. “It’s entirely plausible that the sponges don’t have a nervous system because they’ve lost it because they’re sedentary filter feeders and they don’t need a complex nervous system,” he says. “Simplification and loss happens all the time.”
Sponges wouldn’t be unique in having lost their neurons. Telford points to the example of myxozoans, which are some of the world’s smallest animals and closely related to jellyfish and sea anemones. The common ancestor of these three animals almost certainly had a nervous system, but myxozoans lost theirs at some point in the deep evolutionary past.
The picture is also clouded by the fact that ctenophore neurons are very strange—so strange, in fact, that it might not come as a surprise that neurons in ctenophores emerged independently of neurons in other animals. Another recent paper found that much of the nervous system in ctenophores consists of neurons without synapses, a feature that has not been confirmed to exist anywhere else in the animal kingdom. “There’s no other example of such an extreme variant of a nervous system,” Arendt says. “But there are many examples where nervous systems get reduced and get very simple,” he adds.
Leslie Babonis, an assistant professor of ecology and evolutionary biology at Cornell University, who studies the origin of novel animal traits, can imagine scenarios where these strange neurons still evolved from the same precursor to neurons in other lineages. “There is a lot of evidence to suggest that neurons evolved once in the common ancestor of all animals and that each lineage ... has modified those neurons in really complex and different ways.” At the same time, “it also just challenges our worldview that these animals would give up these important things” such as neurons, she says.
No consensus has emerged. “We need to know more, I think, about nerves and nerve cells and what the precursors to those were,” Telford says.
Indeed, disentangling the evolutionary history of neurons may require addressing some of the basics of how neurons arose in the first place. Biologists have not settled on a model for how neurons could have evolved once—let alone twice. A leading contender has been the “chemical brain hypothesis, sometimes called the “neurosecretory network hypothesis,” which suggests that the precursors to neurons were cells that relied solely on chemical messaging to send signals through an organism. The chemical brain hypothesis got a big boost this past September, thanks to obscure animals called placozoans. Placozoans are invisible without the aid of a microscope. They’re ocean-dwellers, like ctenophores, but they’re only a few cell layers thick, and their bodies are amorphous. Unlike ctenophores, however, placozoans have no neurons. Instead they largely rely on specialized peptidergic cells, which release or respond to short chains of amino acids, to pilot their tiny bodies using only chemical signaling.
The peptidergic cells in placozoans aren’t neurons. They don’t use electrical impulses, and their messaging to nearby cells is restricted to sending signals to other cells—unlike neurons, which can both send and receive them. But a new analysis has found that peptidergic cells have some eerie genetic parallels to neurons and contain proteins associated with physical structures surrounding synapses in nervous systems. This suggests a blueprint for how animal neurons might have evolved and bolsters previous work in establishing a link between chemical secretory cells and nerve cells.
While this research supports the chemical brain hypothesis, it doesn’t rule out other models for neuron evolution. Another framework from the mid-20th century that Arendt has termed the “contractile network hypothesis” posits that neurons were once a part of hypothetical “neuromuscular cells” that may have integrated the functions of both muscles and neurons. Importantly, the chemical brain and contractile network hypotheses aren’t mutually exclusive.
“Neurons, even within one evolutionary lineage, might have two origins,” Arendt says. “Maybe both are true but happen at different positions in the body.” Even different types of synapses within our brains have different origins. It may turn out that many components of our nervous system may have evolved more than once—even if neurons in animals can be traced to a single ancestor.