On a clear September day in 2015, after 10 years of working to get funding, my colleague Kerry Key and I stepped aboard the R/V Langseth, a research ship docked at the Woods Hole Oceanographic Institution in Massachusetts. We were about to lead a 10-day expedition to map a deposit of fresh water, size unknown, hidden 100 meters (about 330 feet) under the rocky seafloor.
Back in the 1960s the U.S. Geological Survey had drilled a series of vertical boreholes off the New Jersey coast, looking for sand deposits and other resources. They unexpectedly struck fresh water, which was baffling. Years later researchers obtained water samples from the same location and analyzed the chemistry, finding to their surprise that the liquid was a mix of recent rainwater and seawater. Rainwater, 65 kilometers (40 miles) out to sea—under the seafloor?
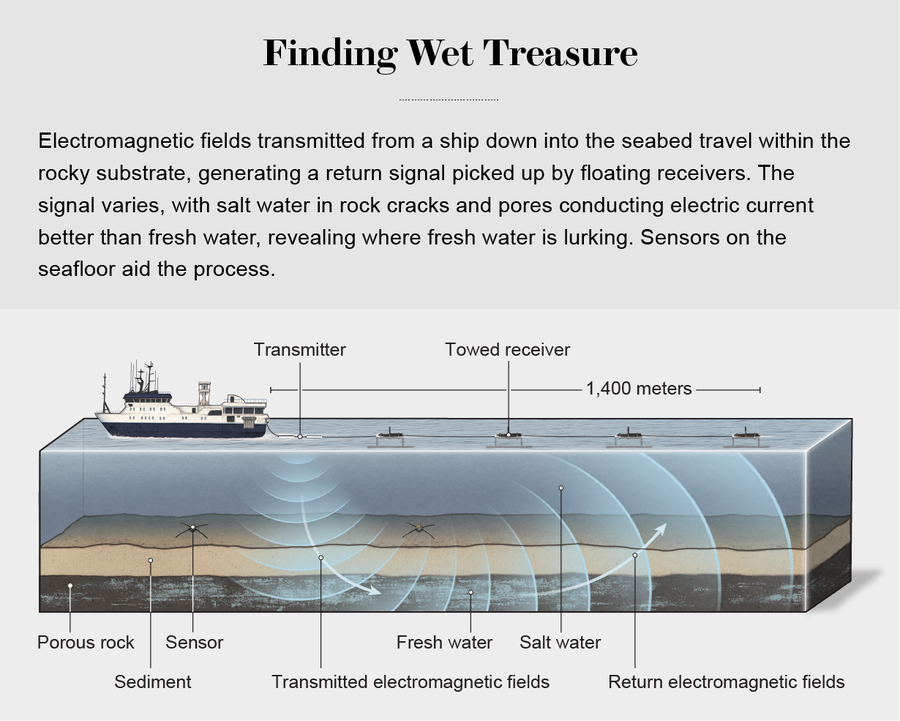
That's where we were headed. Once the R/V Langseth was in position, we spooled out a long, floating line that held a special transmitter. It sent electromagnetic fields hundreds of meters down through the ocean and into the seabed. The fields passed through the seafloor and created secondary, return signals captured by other sensors on the line. We slowly towed the array for 130 kilometers over the region where drilling had been done. We also dropped instruments that sank to the bottom and recorded the signals from our transmitter, as well as naturally occurring electromagnetic fields. We could use all these readings to create an image of what was underneath the seafloor. Once we had completed the survey off New Jersey, we sailed up toward Martha's Vineyard—where researchers had suggested there might also be fresh water—and ran a long sensing profile there, too.
It took us months to process all the data. When we published our results in 2019, we made a stir. One media headline summed up the excitement: “Mysterious Freshwater Reservoir Found Hidden beneath the Ocean.” True. But how big was it? How did it get there? And how common are these offshore underground deposits? We didn't know.
Other questions nagged us. Only about 2.5 percent of all the surface water on this ocean planet is fresh. As the global population grows toward an estimated 10 billion people by 2100, the stresses on our water supply will increase—especially in coastal regions, where 30 percent of the U.S. population now lives. Climate change is also altering rainfall patterns, pollution is compromising extant bodies of water, and agriculture and development are sucking underground reservoirs dry. Could large, hidden reservoirs only a few dozen kilometers out to sea save lives and help irrigate dry crops? Do such reservoirs exist around the world in places where water scarcity is already a huge challenge? If so, could we tap these surprising deposits safely and economically? Our discovery prompted further studies, including recent surveys off San Diego, Hawaii, New Zealand and Malta that are starting to provide answers.
Buried at Sea
Records of fresh water being found offshore go as far back as the 1800s. Fishers off Florida have occasionally reported “boils” of water on the ocean's surface, which they assume leaked upward from below. In some cases, they sampled the water and it did not taste salty; fresh water is less dense than seawater, so it rises.
In 1996, two years after I started at Woods Hole, I was on a small chartered research vessel with six colleagues offshore of Eureka, Calif., the coastline still visible in the distance. We were using a new seafloor-surveying system that had been built at the Pacific Geoscience Center in Canada to map sediments. Our study was part of a large program looking at how flooding rivers that flow to the shoreline disperse sediment into the sea, and our equipment was measuring the amount of seawater in sediment to depths of about 30 meters. It used electromagnetic sensing, a technique that was on the fringes of marine geophysics.
In one area where all other data made us think we should see fine-grained, muddy sediments with high saltwater content, we saw a signal that insinuated the opposite: the reading suggested fresh water that extended for about 50 square kilometers, a sign that groundwater might be leaking from below the shore and oozing through cracks and faults extending into the seafloor. The discovery made us realize that electromagnetic sensing could detect fresh water hiding anywhere under the sea.
A continent does not stop at its shoreline; it extends well offshore as a rocky underwater shelf. The shelf ends at a steep slope that transitions sharply to deep oceanic seafloor. The rock and sediments that make up the world's continental shelves are not dry. Some rocks crack, allowing seawater to penetrate. And most shelves are covered by layers of sedimentary rock, which are like hard sponges with small, interconnected, water-filled pores.
Sediments at or just below the seafloor are typically 40 to 50 percent porous. The weight of the ocean above pushes water down into the sediment as far as it can go. Geoscientists still debate the maximum depth, but it can be at least several kilometers, although the seepage decreases rapidly with depth as the increased pressure closes up cracks and pore spaces. The rock's permeability—the ease with which water can flow through it—depends on how extensively its various pores are interconnected.
Because the shelf is a continuation of the continent, models of groundwater flow in land along the northeastern U.S. coast suggest there could be substantial amounts of fresh water hidden within the rocks and sediments below the continental slope's seafloor. But there are competing hypotheses about how such water might get there—and remain there.
On land, subsurface water is stored in geologic layers of water-bearing rock called aquifers. Some aquifers are shallow and can be replenished by rainfall. Others are much deeper and hold water that has been in place for thousands of years, perhaps left there by glaciers during the last ice age. The composition of aquifers varies across regions, from limestone layers below Florida to more sedimentary layers in the Northeast. Groundwater—the fresh water contained in aquifers—makes up roughly 90 percent of the total available fresh water in the U.S., even when we factor in rivers and lakes. About 25 percent of the water consumed in the U.S. is pumped from aquifers through private or municipal wells.
Off the U.S. East Coast the continental shelf extends anywhere from close to shore to more than 300 kilometers out to sea. Perhaps not surprisingly, the geologic layers that form aquifers under land do not stop at the shoreline; they often extend outward as part of the shelf.
When rain falls on coastal land, it can percolate down into an aquifer and through highly permeable rocks, traveling under and across the shoreline and eventually out to the seabed. For this long-distance flow to occur and for the water to remain fresh, there needs to be a cap over the marine aquifer—a layer that is not permeable, usually of compacted clay-rich sediment. Clay is paradoxical: it can hold a lot of water when loose, but when it is compacted it becomes almost impervious. This cap prevents the less dense fresh water from rising up to the seafloor.
An entirely different mechanism could also leave fresh water under the seafloor. During past ice ages, giant ice sheets and glaciers grew, soaking up large volumes of ocean water. Sea level was much lower, and long sections of continental shelves were exposed as land open to the elements. During the last ice age, roughly between 12,000 and 20,000 years ago, rain falling on these areas could have percolated down into the subsurface, just as it does onshore today. If that water flowed underneath a cap, it could have remained trapped as the ice sheets later melted and sea levels rose again. Yet another model posits that the great weight of the ice sheets pushed fresh water deep into the subsurface and below caps.
Fresh or Salty
Figuring out how a specific reservoir formed—whether it is connected to aquifers on land and how extensive it may be—requires a lot of sensing. Drilling provides samples, but it is expensive and limited to isolated spots. What had been missing until our cruise on the R/V Langseth was a relatively inexpensive, easy-to-use technique that could cover large areas of seafloor.
In the 1970s and 1980s researchers began developing electromagnetic instruments to measure properties of the seafloor, motivated in part by the U.S. Navy's interest in long-distance submarine communications. Through the 1980s and 1990s “controlled source electromagnetic” (CSEM) sensing slowly became more sophisticated. In the late 1990s and early 2000s the petroleum industry began using the technology to detect subsurface oil, which drove significant improvements in the instrumentation available to researchers.
CSEM sensing basically measures how well the seafloor conducts electric current. In the continental shelf, electrical conductivity is controlled by the amount of seawater in pores and cracks, as well as the salinity and temperature of that seawater. The sodium and chloride ions in salt are charge carriers that enhance conductivity, so salt water conducts better than fresh water. A section of ocean floor infused with seawater will conduct current better than a section infused with less saline water. CSEM can measure the differences with fairly high precision.
During our cruise the four receivers on the tow line were 600 to 1,400 meters behind the ship. They measured the electric field generated by the transmitter near the ship, as well as an induced electric field that was detected as it returned from the seafloor substructure. The farther back the receiver, the deeper it could look into the subsurface. That information, along with data about Earth's naturally occurring electric and magnetic fields from the instruments we dropped on the seafloor, allowed us to clearly show that there are submarine freshwater aquifers off New Jersey and Martha's Vineyard.
We still have no good idea about the extent or volume of fresh water, however. Although CSEM conductivity measurements are sensitive to the salinity of pore water, they are also affected by the porosity of the seafloor—how much water is present in a given volume. A rock with high porosity that is less conductive (fresher water) can have the same reading as a rock with low porosity that conducts current well (saltier water). For our CSEM surveys off New Jersey, we used samples of sediment from the drill holes and samples of the pore water to calibrate our models. Salinity is expressed in grams of dissolved salts per liter. The salinity of seawater is around 35. Water with salinity between 1 and 10 is considered brackish. Anything less than 1 is considered fresh. Pore water salinities off New Jersey and Martha's Vineyard range between 0.2 and 9.0.
We have no data for the seafloor between those places, so we do not know whether the two hidden bodies of water are connected or, if so, how. We think there might be fresh water underneath the entire New England shelf, based on surveys and models of aquifers onshore. The water off Martha's Vineyard may have been left there by glaciers more than 12,000 years ago. The water off New Jersey seems to originate in part from rainfall on land. A large team is making plans for scientific drilling off Martha's Vineyard next year, and that work will provide chemical analyses that could help us figure out how long the water has been hiding there.
Farther south along the Eastern Seaboard, the coastal geology transitions to mostly limestone; the movement of underground water there may be different again. To decipher what is happening, we would need much more CSEM surveying, perhaps augmented by drilling in select locations, which would be a costly undertaking. Surveying the transition from land to sea—to find possible water flows from land aquifers to ocean deposits—is challenging. It would require towing a long array in shallow coastal waters with heavy surf and busy boat traffic, as well as data collection with similar sensors on shoreline land. Although the U.S. East Coast is not under significant water stress compared with other parts of the world, the region is relatively well studied and offers perhaps the best opportunity to understand the various processes involved in offshore groundwater transport and storage.
As I mentioned earlier, other experiments have been conducted since our cruise—some in places that have very different geologic settings. A 2018 survey off Hawaii, using much the same equipment as we used, found clear evidence of rock containing fresh water several hundred meters under the seafloor. Unlike New Jersey, Hawaii is built from volcanic rock, which has relatively high permeability. The assumption, not proved yet, is that the submarine aquifers are created by underground runoff from places on land. Hawaii depends on precipitation for its water supply, so understanding how its water may be lost to the ocean through subsurface routes is important.
Temptation to Tap
Interest in finding offshore freshwater deposits has risen significantly in the past few years, notably in regions where freshwater supplies are scarce. Our best guess at how much is trapped within roughly 150 kilometers of seashores worldwide is about one million cubic kilometers. For reference, New York City consumes about 1.4 cubic kilometers a year. Our guess is based mostly on extrapolation from onshore drill holes, as well as on the few offshore surveys so far.
No one has designed a detailed system to tap a submarine aquifer. Tor Bakken of SINTEF Energy Research in Norway and his colleagues described a general system based on oil-drilling technology. A jack-up rig (basically a platform on legs) or a barge would be anchored above a submarine freshwater aquifer. Engineers would drill into the reservoir, and water would flow through a pipeline on the seafloor to a processing plant onshore. The plant would desalinate the water, probably using reverse osmosis, a common filtering technique. Bakken estimated that this process would be slightly cheaper than desalination of seawater, depending on how salty the “fresh water” is. The desalination, which is energy-intensive, would account for a much larger percentage of the total cost than drilling or pumping the water along the pipeline.
To decide whether to exploit any given offshore water supply, we would need to understand how groundwater finds its way into that patch of seafloor to begin with. Imagine a submarine aquifer that isn't connected to any water-conducting structures under the shoreland. The fresh water is surrounded by sediments containing seawater. As soon as someone started pumping the fresh water out, seawater could flow into the void, mixing with the remaining fresh water and raising its salinity. And once the fresh water is extracted, it won't get replenished.
Pumping water from a submarine aquifer that is connected through a geologic formation to an onshore aquifer could also be risky. Any submarine aquifer would be at least slightly brackish, and pumping could mix the waters, which might reduce the freshness of the land aquifer. Modeling also suggests that excessive pumping of offshore reservoirs could drain the onshore water supplying them and even lead to land subsidence.
Between September 2019 and September 2020 researchers using CSEM sensing showed that brackish groundwater within the San Diego Formation, a big underground supply of water for the city, was connected to a submarine aquifer offshore by Coronado Island. Yet the geology of the region is complex, with a number of faults, which could make tapping a submarine aquifer seem less worthwhile. The U.S. West Coast has many geologic faults that could channel groundwater offshore but that could also allow saltwater intrusion onshore if there were excessive pumping. This would seem to be the case for San Diego.
All municipalities have a water-supply strategy, usually involving a range of potable water sources, as well as conservation. Some water-stressed regions, including some entire countries, are already desalinating seawater. The process is expensive and, if the machinery is powered by fossil fuels, emits greenhouse gases. Before a locality considered drilling for submarine fresh water, it might consider groundwater that had been dismissed in the past because it was brackish; it might be less salty than the submarine aquifer. San Diego and El Paso, Tex., are already desalinating brackish groundwater. Another issue could be which country has the right to draw from an offshore aquifer that lies across an ocean boundary between two adjacent nations.
Conservation is also important. Everything on Earth's continents and oceans is connected. Onshore groundwater that flows through the subsurface and offshore brings nutrients and chemicals that sustain delicate marine communities in places along the continental slope. We cannot yet predict the environmental consequences of using offshore groundwater as a resource.
Scientists have confirmed only a small number of submarine freshwater aquifers. There could be many more—small, large, refreshed by groundwater or isolated by ice ages. Community efforts are springing up, particularly in Europe, to explore the possibilities. More surveys will gradually solve the mystery—and reveal more surprises. Mapping in 2022 in the Mediterranean Sea around Malta showed an offshore reservoir probably fed by onshore groundwater. The data and modeling concluded that there may be one cubic kilometer of fresh water offshore, enough to supply the population of the Maltese Islands for 75 years. But the modeling also showed that climate change will lessen future rainfall there, reducing offshore groundwater by 38 percent over the next 80 years or so.
We have a lot to learn. Drilling south of Martha's Vineyard next year will tell us much more about how stores of fresh water under land and sea might connect. The more we investigate, the more we will understand about how these hidden treasures are formed, and the better we will be able to predict where we might find them.

.png?w=900)